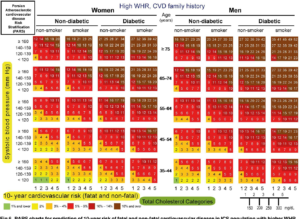
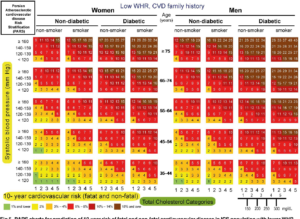
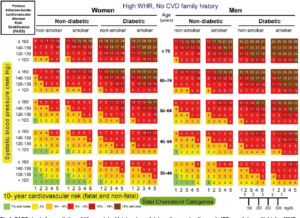
| page number |
Titel |
Description |
Symbol |
| 2 |
Consent letter |
Informed Consent to participate in the plan |
|
| 2 |
General information |
|
|
| 2 |
ID |
Specifications related to the sample |
ID1-ID82 |
| 2 |
Interviewer |
Specifications related to the questioning |
ID9-ID13 |
| 3 |
Nutrition |
|
|
| 3 |
N practice |
|
|
| 3_6 |
Consumption a variety of food |
Yes:1 No:2 |
Np69c-Np178c |
| 3_6 |
Period |
Daily:1 Weekly:2 Monthly:3 Yearly:4 |
Np69d-Np178c |
| 3_6 |
Load |
Number |
Np69n-Np178n |
| 3_6 |
Amout per use |
Number |
Np69q-Np178n |
| 6 |
Drink tea |
Glass or Cup |
Np128n |
| 6 |
Period |
Daily:1 Weekly:2 |
Np128d |
| 6 |
Drink Coffee |
Glass or Cup |
Np129n |
| 6 |
Period |
Daily:1 Weekly:2 |
Np129d |
| 6 |
Consuming which substance with tea or coffee |
Sugar loaf :1 Candy( polki):2 Candy ( nabat):3 sugar:4 other:5 |
Np130 |
| 6 |
Number of consumption |
number of /sugar loaf,polki – match box of nabat- tabelspoon of shgar |
Np130n |
| 6 |
Consuming salt with food |
Never :1 For low salt food:2 Always before meals:3 |
Np134 |
| 6 |
Your taste in terms of salt |
Salty:1 Low salt:2 No salt: 3 Ordinary:4 |
Np135 |
| 6 |
| Eating food prepared at work or school |
|
Yes:1 No:2 |
Np136 |
| 6 |
Which meal? |
How many lunch times a week? |
Np137 |
| 6 |
Which meal? |
How many dinner times a week? |
Np138 |
| 6 |
Change in diet during the past year? |
Reduction of fat and oil: yes:1 No:2 |
Np139 |
| 7 |
Change in diet during the past year? |
Substituting liquid oil instead of solid oil: yes:1 No:2 |
Np140 |
| 7 |
Change in diet during the past year? |
Increase vegetable consumption: yes:1 No:2 |
Np141 |
| 7 |
Change in diet during the past year? |
Reduce the amount of sugar: yes:1 No:2 |
Np142 |
| 7 |
Change in diet during the past year? |
Reduce the amount of salt: yes:1 No:2 |
Np143 |
| 7 |
Change in diet during the past year? |
Eating bread instead of rice: yes:1 No:2 |
Np144 |
| 7 |
Change in diet during the past year? |
Reduce consumption of prepared food: yes:1 e No:2 |
nNp4 |
| 7 |
Regular consumption of vitamin pills, mineral salts or food or bodybuilding supplements? |
Yes:1 No:2 |
Np184 |
|
The name of the supplement or vitamin |
_ |
Np185 |
| 7 |
| Type of supplement or vitamin |
|
injectable:1 edible:2 |
Np185a |
| 7 |
Use of disposable containers |
|
Np186 |
| 7 |
If yes, its type? |
Ordinary plastic containers:1 Lacquered dishes:2 Paper containers:3
Vegetable dishes:4 |
Np187 |
| 7 |
|
| Daily:1 Weekly:2 Monthly:3 |
|
Np188 |
| 7 |
Number of times used |
number |
Np188a |
| 7 |
Reusing disposable containers |
|
Np189 |
| 7 |
|
| Daily:1 Weekly:2 Monthly:3 |
|
Np190 |
| 7 |
Number of times used |
number |
Np190a |
| 7 |
If you see black mold،I remove the mold and use the rest of the food |
|
Np191 |
| 7 |
| if you see black mold ,remove the mold by washing and use the rest of the food ingredients |
|
|
Np192 |
| 7 |
| I throw away the food completely |
|
Yes:1 No:2 |
Np193 |
| 7 |
How do you like tea? |
Hot:1 lukewarm:2 cold:3 |
Np194 |
| 7 |
| Do you eat leftover food? |
|
Yes:1 No:2 |
Np195 |
| 7 |
|
| Daily:1 Weekly:2 Monthly:3 |
|
Np196 |
| 7 |
|
number |
Np196a |
| 7 |
Do you use smoked food? |
Yes:1 No:2 |
Np197 |
| 7 |
|
| Daily:1 Weekly:2 Monthly:3 |
|
Np198 |
| 7 |
|
number |
Np198a |
| 8 |
Stress |
|
|
| 8 |
S.GHQ |
|
|
| 8 |
Health status in the last month (Positive feelings and ability) |
More than usual:1 As usual:2 Less than usual:3 Much less than usual:4 |
Sg1- Sg6 |
| 8 |
| Health status in the last month (Negative feelings and disability) |
|
Never:1 As usual:2 More than usual:3 A lot more than usual:4 |
Sg7-Sg12 |
| 9 |
Cs. Chronic Stressor |
|
|
| 9 |
Stressful events around the person |
Yes:1 No:2 |
|
| 9_10 |
If there is stress, how much is its effect on their life? |
Very low:1 Low:2 medium:3 Much:4 very much:5 |
Cs1-Cs50 |
| 11 |
Methods of coping with stress |
|
|
| 11 |
S.Coping strategies |
|
|
| 11 |
Which items have you used to deal with stressful situations during mental stress? |
mostly:1 sometimes:2 Never:3 |
Sc13-Sc42 |
| 12 |
cigarettes |
|
|
| 12 |
Smoking |
|
|
| 12 |
Do you have a smoking friend? |
yes :1 No:2 |
Sm1 |
| 12 |
| Do you have a hookah friend? |
|
yes:1 No:2 |
gsm1 |
| 12 |
| Do you have a smoker in your family? |
|
yes:1 No:2 |
Sm2 |
| 12 |
| Proportion of smokers in your family |
|
Father:1 Mother:2 sister or brother:3 other:4 |
Sm3 |
| 12 |
| What do you think about a doctor smoking? |
|
It depends on the person:1 She/He should not smoke:2 I don’t care/ Ihave no opinion:3 |
Sm13 |
| 12 |
| What do you think about a teacher or Professor smoking? |
|
| It depends on the person:1 She/He should not smoke:2 I don’t care/ Ihave no opinion:3 |
|
Sm14 |
| 12 |
| What do you think about a athlete smoking? |
|
| It depends on the person:1 She/He should not smoke:2 I don’t care/ Ihave no opinion:3 |
|
Sm15 |
| 12 |
| What do you think about a actress smoking? |
|
| It depends on the person:1 She/He should not smoke:2 I don’t care/ Ihave no opinion:3 |
|
Sm16 |
| 12 |
| What do you think about a Girl or woman smoking? |
|
| It depends on the person:1 She/He should not smoke:2 I don’t care/ Ihave no opinion:3 |
|
Sm17 |
| 12 |
| What do you think about smoking an employee smoking in her/his workplace? |
|
| It depends on the person:1 She/He should not smoke:2 I don’t care/ Ihave no opinion:3 |
|
Sm18 |
| 12 |
| What do you think about smoking Taxi driver in the presence of passengers? |
|
| It depends on the person:1 She/He should not smoke:2 I don’t care/ Ihave no opinion:3 |
|
Sm19 |
| 12 |
| What do you think aboutsmoking Shopkeeper at her/his workplace(feminine)? |
|
| It depends on the person:1 She/He should not smoke:2 I don’t care/ Ihave no opinion:3 |
|
Sm20 |
| 12 |
| What do you think aboutsmoking A person who enters your workplace? |
|
| It depends on the person:1 She/He should not smoke:2 I don’t care/ Ihave no opinion:3 |
|
Sm21 |
| 12 |
| What do you think about smoking A person who enters your home |
|
| It depends on the person:1 She/He should not smoke:2 I don’t care/ Ihave no opinion:3 |
|
Sm22 |
| 12 |
| What do you think about a doctor smoking hookah? |
|
It depends on the person:1 She/He should not smoke hookah:2
There is no problem/I have no opinion:3 |
|
gSm3 |
| 12 |
| What do you think about a teacher or Professor smoking hookah? |
|
It depends on the person:1 She/He should not smoke hookah:2
There is no problem/I have no opinion:3 |
|
gSm4 |
| 12 |
| What do you think about a athlete smoking hookah? |
|
It depends on the person:1 She/He should not smoke hookah:2
There is no problem/I have no opinion:3 |
|
gSm5 |
| 12 |
| What do you think about a actress smoking hookah? |
|
It depends on the person:1 She/He should not smoke hookah:2
There is no problem/I have no opinion:3 |
|
gSm6 |
| 12 |
| What do you think about a Young Girl smoking hookah? |
|
It depends on the person:1 She/He should not smoke hookah:2
There is no problem/I have no opinion:3 |
|
gSm7 |
| 12 |
| What do you think about a Young Boy smoking hookah? |
|
It depends on the person:1 She/He should not smoke hookah:2
There is no problem/I have no opinion:3 |
|
gSm8 |
| 12 |
| What do you think about a old man and old woman smoking hookah? |
|
It depends on the person:1 She/He should not smoke hookah:2
There is no problem/I have no opinion:3 |
|
gSm9 |
| 12 |
| What do you think about smoking hookah At home and with the family? |
|
It depends on the person:1 She/He should not smoke hookah:2
There is no problem/I have no opinion:3 |
|
gSm10 |
| 12 |
| What do you think about smoking hookah only in the traditional coffee house and tea house? |
|
It depends on the person:1 She/He should not smoke hookah:2
There is no problem/I have no opinion:3 |
|
gSm11 |
| 12 |
| What do you think about smoking hookah In chic restaurants and hotels? |
|
It depends on the person:1 She/He should not smoke hookah:2
There is no problem/I have no opinion:3 |
|
gSm12 |
| 12 |
| What do you think about smoking hookah Home Alone? |
|
It depends on the person:1 She/He should not smoke hookah:2
There is no problem/I have no opinion:3 |
|
gSm13 |
| 12 |
| What do you think about smoking hookahIn a traditional coffee house and tea house with friends? |
|
It depends on the person:1 She/He should not smoke hookah:2
There is no problem/I have no opinion:3 |
|
gSm14 |
| 12 |
Have you ever used tobacco (cigarette/pipe/hookah etc…? |
Yes:1 No:2( If No refer to question 131/page 13) |
|
| 12 |
| Do you currently use tobacco (cigarettes/pipes/hookahs etc…)? |
|
Yes:1 No:2( If No refer to question 102/page 13) |
|
| 12 |
|
|
|