Comparison of laboratory-based and non-laboratory-based WHO cardiovascular disease risk charts: a population-based study
Abstract
Background: Determining the risk of Cardiovascular Disease (CVD) is a necessity for timely preventive interventions
in high-risk groups. However, laboratory testing may be impractical in countries with limited resources. This study
aimed at comparison and assessment of the agreement between laboratory-based and non-laboratory-based WHO
risk charts models.
Methods: This study was performed using the baseline data of 8138 participants in the pars cohort study who had
no history of CVD and stroke. The updated 2019 WHO model was used to determine the 10-year fatal and non-fatal
CVD risks. In general, there are two types of new WHO risk prediction models for CVD. The scores were determined
based on age, sex, smoking status, diabetes, Systolic Blood Pressure (SBP), and total cholesterol for the laboratory-
based model and age, sex, smoking status, SBP, and Body Mass Index (BMI) for the non-laboratory-based model. The
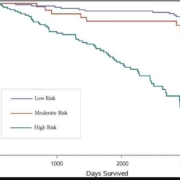
agreement of these two models was determined via kappa statistics for the classified risk (low: < 10%, moderate:
10–< 20%, high: ≥ 20%). Correlation coefficients (r) and scatter plots was used for correlation between scores.
Results: The results revealed very strong correlation coefficients for all sex and age groups (r = 0.84 for
males < 60 years old, 0.93 for males ≥ 60 years old, 0.85 for females < 60 years old, and 0.88 for females ≥ 60 years old).
In the laboratory-based model, low, moderate, and high risks were 76.10%, 18.17%, and 5.73%, respectively. These
measures were respectively obtained as 77.00%, 18.08%, and 4.92% in the non-laboratory-based model. Based on risk
classification, the agreement was substantial for males < 60 years old and for both males and females aged ≥ 60 years
(kappa values: 0.79 for males < 60 years old, 0.65 for males ≥ 60 years old, and 0.66 for females ≥ 60 years old) and
moderate for females < 60 years old (kappa = 0.46).
Conclusions: The non-laboratory-based risk prediction model, which is simple, inexpensive, and non-invasive, classi-
fies individuals almost identically to the laboratory-based model. Therefore, in countries with limited resources, these
two models can be used interchangeably.