Principal Investigator:
Fereidoun Azizi
Professor of Internal Medicine and Endocrinology
Director of Research Institute for Endocrine Sciences
Shahid Beheshti University of Medical Sciences
Start date
The Tehran Lipid and Glucose Study (TLGS) as the first Community –based large-scale long-term cohort study in I.R.Iran was designed in 1998 and initiated in 1999
Goals
To study the incidence of non-communicable diseases and the association between different exposures and outcomes.
Study population
Nearly 27,500 people (7,146 households) aged ≥3 years living in District 13 under coverage by three health centers, the Leylatolghadr (7,858), Mohammadian (8,932), and Salavati (10,761) centers, where their health records are available.
Sampling method and sample size
The sample size was determined to be 14,280 considering the following items:
- Confidence interval of 95%,
- Study power of 80%,
- Predicted prevalence rates of 30% for dyslipidemia in people under 30 years old, and 45% for those 30 years old and over,
- Attrition rate estimation of 20%,
- Design effect of 1.25,
- Seven age groups and two genders.
Sample Selection
Using cluster random sampling, samples were selected as follows:
- A list of all households under coverage by the three health centers was prepared.
- Households were specified according to each health center.
- Proportions of households under coverage by each health center to the total number of households in the three health centers were determined.
Lists of all households selected were prepared and their addresses were determined.
Data Collection
- Invitation
All individuals were invited for interviews and medical examinations to the TLGS unit. Social workers first familiarized them at home with the study objectives and invited them to participate in the study at the second visit; they were asked to sign a written consent and bring it to the TLGS unit following the first visit. At TLGS unit they underwent:
- Interview
Each interview was conducted by means of a structured questionnaire to collect demographic data such as personal, educational, occupational, medical, and residential details.
- Medical examination
In this stage, trained physicians collected data on the medical history, blood pressure, peripheral pulse, and thyroid exam. Trained technicians obtained anthropometrical data such as height, weight, hip, and waist sizes. ECG was taken from those aged 30 years and over.
- Blood sampling
Blood samples were drawn after 10-12 hours of fasting and the glucose tolerance test (2hPG) was performed in those aged 20 years and over.
- Laboratory procedures
After admission and documentation of personal characteristics and determination of a computer code, a 10-ml sample of venous blood was collected. The samples were kept for one and half hours in normal conditions of the lab. The samples were then centrifuged to separate the sera; all the tests were done daily and some samples (four micro-tubes) were stored in –70C temperature for future studies.
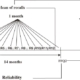
Follow up Methods
Active fallow-up every 3 years to repeat all measurements. Up to now, 6 examinations have been conducted. – Active follow up annually for hospitalized events and death. Up to know, 18 years of follow-up have been completed.
Main Exposures
Demographic, behavioral (e.g. nutrition, physical activity and smoking), medical history, family history, biological (e.g. anthropometric, blood pressure, pulse rate), biochemical (e.g. serum levels of glucose, lipid profile and kidney, liver and thyroid function tests) and genetic factors.
Outcomes
NCDs events especially cardiovascular diseases, diabetes and other metabolic disorders and cancer. Up to Mar 2016 for any death and hospitalization and up to Mar 2017 for all measurements (Phase 6 of the TLGS) including self-reported physician diagnosis, drug history, and diagnoses based on physical exams and lab measurements, e.g. hypertension and diabetes.
Cohort Study
About The Tehran Lipid and Glucose Study (TLGS)
Introduction Video
TLGS Documentation
RATIONALE AND DESIGN Papers
Metadata Dictionary
Gallery
TLGS Timeline
1998
Approval Date
TLGS was designed as the first Community –based large scale long term cohort study in I.R.Iran
1999
Phase I
Phase I was conducted between February 1999 and August 2001.
1999
2001
Phase II
Phase II was conducted between September 2001 and …
2004
Phase III
Milestone Content goes here
2004
2007
Phase IV
Milestone Content goes here
2010
Phase V
Milestone Content goes here
2010
2014
Phase VI
Milestone Content goes here
2018
Phase VII
Milestone Content goes here
2018
News
Selected Publications
Contact
No.24, Aarabi St, Yaman St, Velenjak, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Postal Code: 1985717413, P.O Box: 19395-4763
- Tel: +982122432500 (612, 457)
- Tel: +982122402463
- Fax: +982122416264
Latest News
 Webinar: Shahrekord Prospective Epidemiological Research Studies in Iran (PERSIAN) experienceJune 3, 2024 - 4:22 pm
Webinar: Shahrekord Prospective Epidemiological Research Studies in Iran (PERSIAN) experienceJune 3, 2024 - 4:22 pm Association between latent profile of dietary intake and cardiovascular diseases (CVDs): Results from Fasa Adults Cohort Study (FACS)October 18, 2023 - 11:43 am
Association between latent profile of dietary intake and cardiovascular diseases (CVDs): Results from Fasa Adults Cohort Study (FACS)October 18, 2023 - 11:43 am Association between adherence to the Mediterranean diet with cardiometabolic risk factors: a cross-sectional study on PERSIAN cohort study in FasaSeptember 8, 2023 - 8:09 am
Association between adherence to the Mediterranean diet with cardiometabolic risk factors: a cross-sectional study on PERSIAN cohort study in FasaSeptember 8, 2023 - 8:09 am