Famenin Brucellosis Cohort Study
Hamadan University of Medical Sciences, Infectious Disease Research Center(Brucellosis Research Center)
Principal Investigator:
Fariba Keramat(retired) *
Fatemeh Torkamanasadi **
*
Professor of Tropical and Infectious Diseases
Hamadan University of Medical Sciences
Director, Brucellosis Research Center
**
Assistant Professor of Infectious Disease
Infection Disease Research Center
Shahid Beheshti Medical Educational Center
Health Vice chancellor of Hamadan Universtiy of Medical Sciences
Approval date
11/01/2014
Approved the 617th meeting of the Research Council of the Vice Chancellor for Research and Technology of Hamadan University of Medical Sciences
Announcement session
15-7-2014
session for Announcement and introduction of cohort study
Coordination meeting
15-10-2014
Coordination meeting and profile review
Goals
Stage1: • To determination of brucellosis seroprevalence in Famenin populations. • To Identification and prioritization of risk factors. Stage2: • To determination the seroprevalence of brucellosis in the livestock population. • To identification of human and livestock species • To determine Antibiotic resistance. • To study the Prevalence of microbial contamination of dairy products. • To determine the pattern of disease transmission. • To Intervention to control, reduce, and prevent. Stage 3: • To evaluation of implementer intervention on brucellosis incidence.
Study population
Urban and rural population and livestock in Famenin city ,Hamadan province ,Iran
Sampling method and sample size
Two stage sampling (clusters were selected using systematic sampling) in Famenin city population for serologic investigation in 3161 persons were used. All cases of brucellosis will enroll in the study All brucellosis cases, infected livestock and dairy products in Famenin city will investigate
Starting date
Autumn 2014
Data Collection
The data in this study will be collected in three phases: Phase 1: Research was conducted to determine the serological status of human contamination and to identify and prioritize risk factors, and to record all cases of brucellosis. phase 2: In patients: clinical symptoms, transmission pattern, complications and recurrence, diagnostic and treatment methods, drug resistance will investigate. Investigations will be carried out to determine the serological status of livestock contamination. Educational interventions and vaccination of livestock will be done. Phase 3: Monitoring and intervention studies
Follow up Methods
Monitoring the clinical signs and seroprevalence of brucellosis in humans and livestock populations studied in Famenin during the study period.
Main Exposures
brucellosis Risk factors
Outcomes
Evaluation of subclinical and chronic brucellosis, relapse, complications, and drug resistance in the patients with brucellosis
cohort study
- name
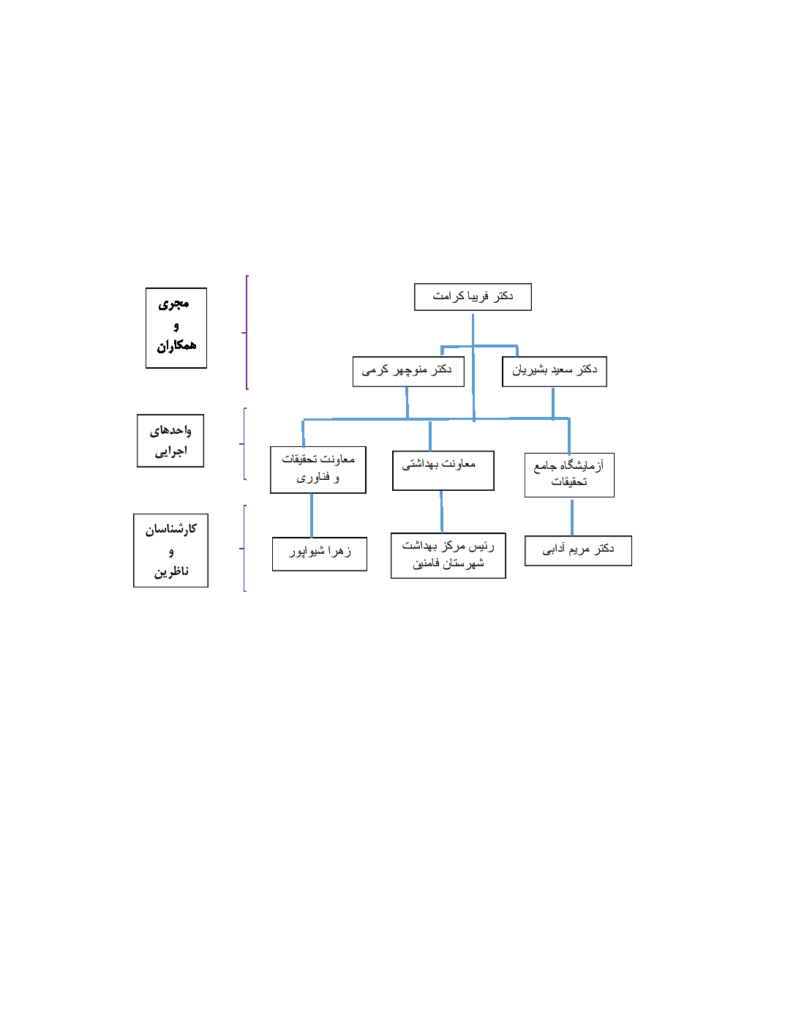
- dr. Fariba Keramat
- dr. Saeed Bashirian
- dr, Manoochehr karami
- dr.Abbas Moghimbeigi
- dr.Mohammad Yousef Alikhani
- dr. Saeed Khakizade
- dr. Maryam Adabi
- dr. Mehran Rajabi
- dr. Mehdi Dinari
- Zahra Shivapour
- position
- Administration Manager
- Executive team member
- Executive team member
- Executive team member
- Executive team member
- Develop executive instructions for blood sampling and quality control
- Head of Comprehensive Research Laboratory
- Head of Famenin Health Center
- Head of Famenin Health Center
- MSC in epidemiology
- name
- Professor of Infectious Diseases
- PhD in Health Education and Promotion
- PhD in epidemiology
- PhD in Biostatistics
- PhD in microbioligy
- Professional Doctor of Laboratory Sciences
- PhD in microbioligy
- General
Physician - General
Physician - supervisor, development instructions, quality control
Rationale and design paper
Keramat F, Karami M, Alikhani MY, Bashirian S, Moghimbeigi A, Adabi M. Cohort profile: Famenin Brucellosis Cohort Study. J Res Health Sci. 2019 Aug 3;19(3):e00453. PMID: 31586374; PMCID: PMC7183558.https://pubmed.ncbi.nlm.nih.gov/31586374/
HBCS Questionnaires
1- Phaz1 1 questionnaireQuestionaire A- PHAZE 1 HBCS
2- Phaze 2 questionnare questionnaire B PHAZE 2 HBCS