Principal Investigator:
Seyed Abbas Motevalian
Professor of Epidemiology, Department of Epidemiology, School of Public Health, Iran University of Medical Sciences
amotevalian@iums.ac.ir, amotevalian@yahoo.com
Approval date
2016/02/16
Starting date
2017/07/04
Goal
Focused on job stressors and socioeconomic gradient to evaluate the prevalence and long-term trends of NCDs and their outcomes (i.e. Death, Sickness Absence, NCD (cardiovascular, cancer, musculoskeletal, psychiatric, and other NCD), Injuries)
Study population
14,000 employees of IUMS or Ministry of Health and Medical Education include:
◦Iran University of Medical Sciences in
- 17 hospitals
- 9 schools
- 7 health networks
◦Ministry of Health and Medical Education
Sampling method and sample size
According to the memorandum of understanding with the Vice-Chancellor of Research and Technology of the Ministry of Health, the sample size of the study is 10,000 people.
It is estimated that the total number of eligible people is about 13,000, and if 75-80 percent of people participate, the sample size will be 10,000 people.
Till now 4886 participants have been recruited.
Data Collection
Follow up Methods
Phone, Annual follow-ups (200,000 person-year) of the cohort were designed to be conducted up to 2032.
Study Waves
- Comprehensive assessments every four years
- Telephone follow-ups each year
- At least 4 waves (16 years)
Main Exposures
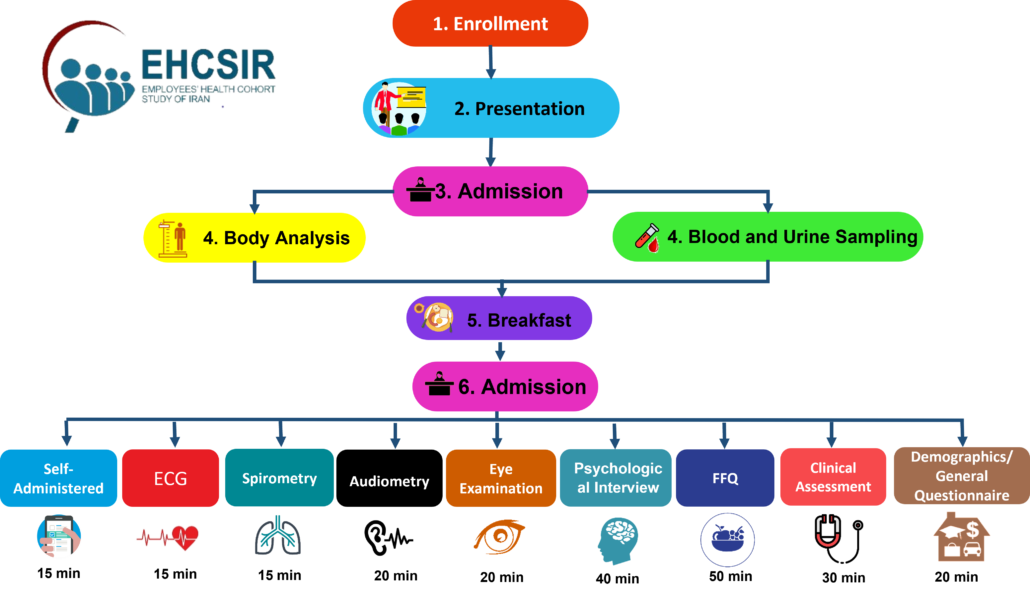
The study collected data on a large number of exposures such as demographics, general health, lifestyle, dietary habits, nutrition and eating habits, employment, social integration, quality of life (e.g., sleep patterns, stress), physical activity, anthropometric indexes, occupational history, living place, family medical history and use of medication, and the number of anthropometric and physiological measures (e.g., respiratory capacity tests, electrocardiograms).
A rich biobank was created by collecting, in all cohort participants, samples from whole blood, serum, plasma, buffy coat and urine. Diseases were ascertained from clinical examinations, biochemical variables, interviews, and linkage with medical records registered in the integrated health information system.
Outcomes
Data Dictionary
Staff
EHCSIR Timeline
FEB 2016
Approval Date
March 2017
Inauguration of the Study Site
March 2017
May 2017
Pre-Pilot
68 participants
July 2017
Pilot
718 participants
July 2017
Feb 2018
Phase I Enrollment
Dec 2019
First Follow up
Dec 2019
Mar 2020
End of Phase I Enrollment
4886 Participants
Dec 2020
Second Follow up
Dec 2020
Jan 2021
Covid-19 Seroepidemiology Study
Feb 2022
Third Follow up
Feb 2022
NEWS
World Health Organization representative: EHCSIR helps redesign work environments based on employees` health indicators
Assessment Process